Urea vs. carbamate groups: a comparative study in a chiral C2 symmetric organogelator†
Abstract
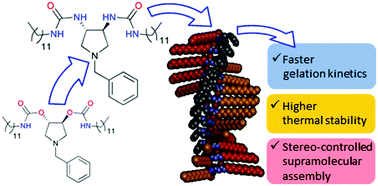
The effect of the replacement of molecular moieties (carbamates vs. urea) that drive self-assembly for two organogelators with an identical C2 symmetric molecular structure is described. The main properties of the gels obtained from the urea-based organogelators are also discussed. The proposed organogelators are chiral molecules and are able to express chirality also at the supramolecular level, thus allowing the employment of electronic circular dichroism to gain insight into the molecular-scale structure of fibrillar aggregates. With the same technique, the behavior of enantiomeric mixtures of the urea-based organogelators was investigated, revealing the occurrence of different self-sorting phenomena at the molecular and supramolecular scale. The urea-based organogelators demonstrated to be more efficient gelators with respect to the carbamate-based analogues, showing a high gel-to-sol transition temperature (up to 66 °C) and a very low minimum gelling concentration (0.85 mg mL−1). This study is a starting point for a deeper investigation of structure/property relationships and, taking into account the peculiar behavior detected for the enantiomeric mixtures, also of self-sorting and molecular recognition phenomena.

 Please wait while we load your content...
Please wait while we load your content...