Remarkable distinctions in the heat-induced phase transition processes of two poly(2-isopropyl-2-oxazoline)-based mixed aqueous solutions†
Abstract
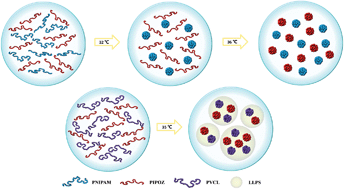
Detailed phase transition processes of poly(2-isopropyl-2-oxazoline) (PIPOZ)/poly(N-isopropylacrylamide) (PNIPAM) and PIPOZ/poly(N-vinylcaprolactam) (PVCL) mixtures in aqueous solution were investigated by DSC, temperature-variable 1H-NMR, optical microscopy and FT-IR spectroscopy measurements accompanied with two-dimensional correlation spectroscopy (2Dcos) and perturbation correlation moving window (PCMW) analytical methods. Through the comparison of these two systems, it is revealed that PVCL chains can interact with PIPOZ chains directly through the polymer–water–polymer cross-linking hydrogen bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯D–O–D⋯O
O⋯D–O–D⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), which induce their transition process as one. However, in the PIPOZ/PNIPAM mixture, the phase transition of the given component (PNIPAM or PIPOZ) is indirectly affected by the presence of the second component, because the strong hydrogen bonds C
C), which induce their transition process as one. However, in the PIPOZ/PNIPAM mixture, the phase transition of the given component (PNIPAM or PIPOZ) is indirectly affected by the presence of the second component, because the strong hydrogen bonds C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯D–N in PNIPAM components forbid the direct connection with PIPOZ, which induces two phase transition processes separately with no liquid–liquid phase separation (LLPS). Additionally, the formation of polymer–water–polymer hydrogen bonds (C
O⋯D–N in PNIPAM components forbid the direct connection with PIPOZ, which induces two phase transition processes separately with no liquid–liquid phase separation (LLPS). Additionally, the formation of polymer–water–polymer hydrogen bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯D–O–D⋯O
O⋯D–O–D⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) is highlighted as the key process in macroscopic LLPS.
C) is highlighted as the key process in macroscopic LLPS.

 Please wait while we load your content...
Please wait while we load your content...