Comparison of LCST-transitions of homopolymer mixture, diblock and statistical copolymers of NIPAM and VCL in water†
Abstract
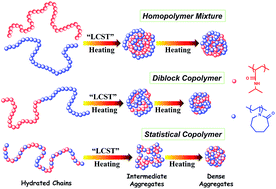
The LCST-transitions of linear, well-defined polymers of N-isopropylacrylamide (NIPAM) and N-vinylcaprolactam (VCL), including a homopolymer mixture, diblock and statistical copolymers, in water are explored and compared by applying turbidity and FTIR measurements in combination with two-dimensional correlation spectroscopy (2Dcos). Only one transition is observed in all polymer systems, suggesting a dependent aggregation of poly(N-isopropylacrylamide) (PNIPAM) and poly(N-vinylcaprolactam) (PVCL) parts in the phase transition processes. With the help of 2Dcos analysis, it is discovered that the hydrophobic interaction among C–H groups is the driving force for simultaneous collapse of the two distinct thermo-responsive segments. Additionally, the delicate differences within the LCST-transitions thereof have been emphasized, where the phase separation temperatures of the homopolymer mixture and the diblock copolymer are close while that of the statistical copolymer is relatively higher. Moreover, both diblock and statistical copolymers exhibit rather sharp phase transitions while the homopolymer mixture demonstrates a moderately continuous one.

 Please wait while we load your content...
Please wait while we load your content...