Membrane protein mobility depends on the length of extra-membrane domains and on the protein concentration†
Abstract
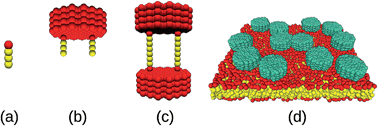
Diffusion of membrane proteins is not only determined by the membrane anchor friction but also by the overall concentration of proteins and the length of their extra-membrane domains. We have studied the influence of the latter two cues by mesoscopic simulations. As a result, we have found that the total friction of membrane proteins, γ, increases approximately linearly with the length of the extra-membrane domain, L, whereas a slightly nonlinear dependence on the total protein concentration, ϕ was observed. We provide an educated guess for the functional form of γ(L, ϕ) and the associated diffusion coefficient. This expression not only matches our simulation data but it is also in favorable agreement with previously published experimental data. Our findings indicate that diffusion coefficients of membrane proteins are not solely determined by the friction of membrane anchors but also extra-membrane domains and the crowdedness of the membrane need to be considered to obtain a comprehensive view of protein diffusion on cellular membranes.

 Please wait while we load your content...
Please wait while we load your content...