Two dimensional inorganic electride-promoted electron transfer efficiency in transfer hydrogenation of alkynes and alkenes†
Abstract
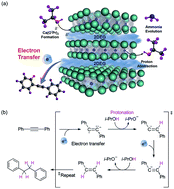
A simple and highly efficient transfer hydrogenation of alkynes and alkenes by using a two-dimensional electride, dicalcium nitride ([Ca2N]+·e−), as an electron transfer agent is disclosed. Excellent yields in the transformation are attributed to the remarkable electron transfer efficiency in the electride-mediated reactions. It is clarified that an effective discharge of electrons from the [Ca2N]+·e− electride in alcoholic solvents is achieved by the decomposition of the electride via alcoholysis and the generation of ammonia and Ca(OiPr)2. We found that the choice of solvent was crucial for enhancing the electron transfer efficiency, and a maximum efficiency of 80% was achieved by using a DMF mixed isopropanol co-solvent system. This is the highest value reported to date among single electron transfer agents in the reduction of C–C multiple bonds. The observed reactivity and efficiency establish that electrides with a high density of anionic electrons can readily participate in the reduction of organic functional groups.

 Please wait while we load your content...
Please wait while we load your content...