Bond-bending isomerism of Au2I3−: competition between covalent bonding and aurophilicity†
Abstract
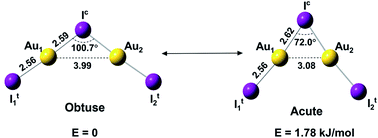
We report a joint photoelectron spectroscopy and theoretical investigation of the gaseous Au2I3− cluster, which is found to exhibit two types of isomers due to competition between Au–I covalent bonding and Au–Au aurophilic interactions. The covalent bonding favors a bent IAuIAuI− structure with an obtuse Au–I–Au angle (100.7°), while aurophilic interactions pull the two Au atoms much closer, leading to an acutely bent structure (72.0°) with an Au–Au distance of 3.08 Å. The two isomers are separated by a small barrier and are nearly degenerate with the obtuse isomer being slightly more stable. At low temperature, only the obtuse isomer is observed; distinct experimental evidence is observed for the co-existence of a combination of isomers with both acute and obtuse bending angles at room temperature. The two bond-bending isomers of Au2I3− reveal a unique example of one molecule being able to oscillate between different structures as a result of two competing chemical forces.


 Please wait while we load your content...
Please wait while we load your content...