Cascade reactions of nitrogen-substituted isocyanates: a new tool in heterocyclic chemistry†
Abstract
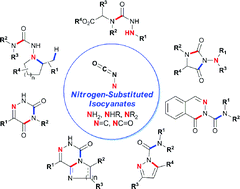
In contrast to normal C-substituted isocyanates, nitrogen-substituted isocyanates (N-isocyanates) are rare. Their high reactivity and amphoteric/ambident nature has prevented the scientific community from exploiting their synthetic potential. Recently, we have developed an in situ formation approach using a reversible equilibrium, which allows controlled generation and reactivity of N-isocyanates and prevents the dimerization that is typically observed with these intermediates. This blocked (masked) N-isocyanate approach enables the use of various N-isocyanate precursors to assemble heterocycles possessing the N–N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O motif, which is often found in agrochemicals and pharmaceuticals. Cascade reactions for the rapid assembly of several valuable 5- and 6-membered heterocycles are reported, including amino-hydantoins, acyl-pyrazoles, acyl-phthalazinones and azauracils. Over 100 different compounds were synthesized using amino-, imino- and amido-substituted N-isocyanates, demonstrating their potential as powerful intermediates in heterocyclic synthesis. Their reactivity also enables access to unprecedented bicyclic derivatives and to substitution patterns of azauracils that are difficult to access using known methods, illustrating that controlled reactivity of N-isocyanates provides new disconnections, and a new tool to assemble complex N–N–C
O motif, which is often found in agrochemicals and pharmaceuticals. Cascade reactions for the rapid assembly of several valuable 5- and 6-membered heterocycles are reported, including amino-hydantoins, acyl-pyrazoles, acyl-phthalazinones and azauracils. Over 100 different compounds were synthesized using amino-, imino- and amido-substituted N-isocyanates, demonstrating their potential as powerful intermediates in heterocyclic synthesis. Their reactivity also enables access to unprecedented bicyclic derivatives and to substitution patterns of azauracils that are difficult to access using known methods, illustrating that controlled reactivity of N-isocyanates provides new disconnections, and a new tool to assemble complex N–N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O containing motifs.
O containing motifs.

 Please wait while we load your content...
Please wait while we load your content...