Light and heat control over secondary structure and amyloid-like fiber formation in an overcrowded-alkene-modified Trp zipper†
Abstract
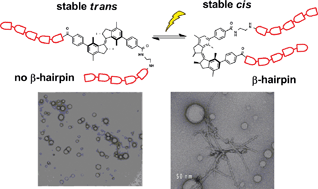
The external photocontrol over peptide folding, by the incorporation of molecular photoswitches into their structure, provides a powerful tool to study biological processes. However, it is limited so far to switches that exhibit only a rather limited geometrical change upon photoisomerization and that show thermal instability of the photoisomer. Here we describe the use of an overcrowded alkene photoswitch to control a model β-hairpin peptide. This photoresponsive unit undergoes a large conformational change and has two thermally stable isomers which has major influence on the secondary structure and the aggregation of the peptide, permitting the phototriggered formation of amyloid-like fibrils.


 Please wait while we load your content...
Please wait while we load your content...