Chiral ion-pair organocatalyst promotes highly enantioselective 3-exo iodo-cycloetherification of allyl alcohols†
Abstract
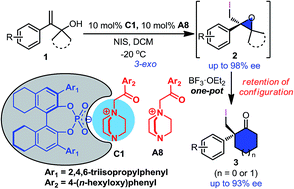
By designing a novel chiral ion-pair organocatalyst composed of chiral phosphate and DABCO-derived quaternary ammonium, highly enantioselective 3-exo iodo-cycloetherification of allyl alcohols was achieved using NIS as a halogen source. Based on this reaction, one-pot asymmetric 3-exo iodo-cycloetherification/Wagner–Meerwein rearrangement of allyl alcohols en route to enantioenriched 2-iodomethyl-2-aryl cycloalkanones was subsequently developed. Due to the participation of adjacent iodine, the Wagner–Meerwein rearrangement of 2-iodomethyl-2-aryl epoxide proceeds with unusual retention of stereoconfiguration.

 Please wait while we load your content...
Please wait while we load your content...