Abstract
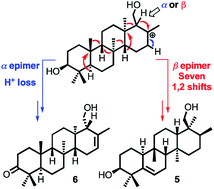
A series of novel sesterterpenes (2–6) have been isolated from the roots of Aletris farinosa and structurally characterized by MS, NMR, and X-ray crystallography in conjunction with computational modeling. Their structures provide new insights into the mechanisms of sesterterpene biosynthesis. Specifically, we propose with support from density functional theory computations that the configuration at a single stereocenter determines the fate of a key tetracyclic carbocationic intermediate, derived from an oxidogeranylfarnesol precursor. Whereas one epimer of the carbocation undergoes H+ elimination to give 6, the other undergoes a spectacular cascade of seven 1,2-methyl and hydride migrations leading to the previously unreported carbon skeleton of 5. Theoretical calculations suggest that the cascade is triggered by substrate preorganization in the enzyme active site.


 Please wait while we load your content...
Please wait while we load your content...