Highly selective hydrogenation of CO2 into C2+ alcohols by homogeneous catalysis†
Abstract
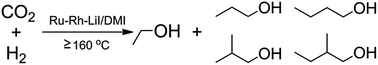
The hydrogenation of CO2 to produce alcohols with two or more carbons (C2+ alcohols) is of great importance, but is challenging. In this work, we found that a Ru3(CO)12/Rh2(CO)4Cl2–LiI system could catalyze the reaction effectively in 1,3-dimethyl-2-imidazolidinone (DMI) under mild conditions. Methanol, ethanol, propanol, 2-methyl propanol, butanol, and 2-methyl butanol were produced in the homogeneous catalytic reaction. The C2+ alcohols could be generated at 160 °C, which is the lowest temperature reported so far for producing C2+ alcohols via CO2 hydrogenation. The selectivity for the C2+ alcohols could be as high as 96.4% at the optimized conditions, which is higher than those reported in the literature. In addition, the catalytic system could be easily recycled. The route of the reaction for forming the C2+ alcohols was discussed on the basis of control experiments.

 Please wait while we load your content...
Please wait while we load your content...