RhI/RhIII catalyst-controlled divergent aryl/heteroaryl C–H bond functionalization of picolinamides with alkynes†
Abstract
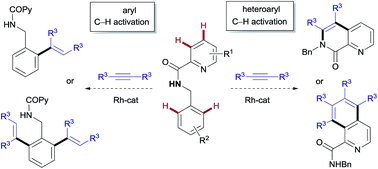
The ability to establish switchable site-selectivity through catalyst control in the direct functionalization of molecules that contain distinct C–H bonds remains a demanding challenge that would enable the construction of diverse scaffolds from the same starting materials. Herein we describe the realization of this goal, namely a divergent heteroaryl/aryl C–H functionalization of aromatic picolinamide derivatives, targeting two distinct C–H sites, either at the pyridine ring or at the arene unit, to afford isoquinoline or ortho-olefinated benzylamine (or phenethylamine) derivatives. This complementary reactivity has been achieved on the basis of a RhIII/RhI switch in the catalyst, resulting in different mechanistic outcomes. Notably, a series of experimental and DFT mechanistic studies revealed important insights about the mechanism of the reaction and reasons behind the divergent regiochemical outcome.


 Please wait while we load your content...
Please wait while we load your content...