Gold(i)-catalyzed cycloisomerization of vinylidenecyclopropane-enes via carbene or non-carbene processes†
Abstract
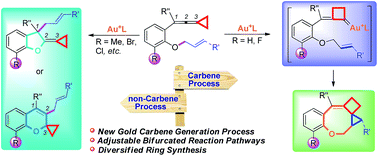
Gold catalyzed cycloisomerization of aromatic ring tethered vinylidenecyclopropane-enes provides a divergent synthetic protocol for the construction of O-containing fused heterocycles through controllable carbene or non-carbene related processes. The carbene induced process features a new amphiphilic strategy to generate a gold carbene via a rearrangement of vinylidenecyclopropane. Whereas, the electronic effect of the ortho-substituents switches the reaction mode onto the non-carbene related process, from which five- or six-membered rings are selectively produced through allyl-migration.

 Please wait while we load your content...
Please wait while we load your content...