Mechanistic insights into the reductive dehydroxylation pathway for the biosynthesis of isoprenoids promoted by the IspH enzyme†
Abstract
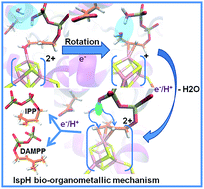
Here, we report an integrated quantum mechanics/molecular mechanics (QM/MM) study of the bio-organometallic reaction pathway of the 2H+/2e− reduction of (E)-4-hydroxy-3-methylbut-2-enyl pyrophosphate (HMBPP) into the so called universal terpenoid precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), promoted by the IspH enzyme. Our results support the viability of the bio-organometallic pathway through rotation of the OH group of HMBPP away from the [Fe4S4] cluster at the core of the catalytic site, to become engaged in a H-bond with Glu126. This rotation is synchronous with π-coordination of the C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3 double bond of HMBPP to the apical Fe atom of the [Fe4S4] cluster. Dehydroxylation of HMBPP is triggered by a proton transfer from Glu126 to the OH group of HMBPP. The reaction pathway is completed by competitive proton transfer from the terminal phosphate group to the C2 or C4 atom of HMBPP.
C3 double bond of HMBPP to the apical Fe atom of the [Fe4S4] cluster. Dehydroxylation of HMBPP is triggered by a proton transfer from Glu126 to the OH group of HMBPP. The reaction pathway is completed by competitive proton transfer from the terminal phosphate group to the C2 or C4 atom of HMBPP.


 Please wait while we load your content...
Please wait while we load your content...