Terpyridine–Cu(ii) targeting human telomeric DNA to produce highly stereospecific G-quadruplex DNA metalloenzyme†
Abstract
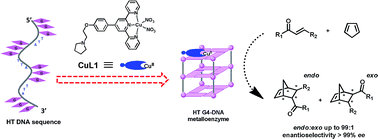
The cofactors commonly involved in natural enzymes have provided the inspiration for numerous advances in the creation of artificial metalloenzymes. Nevertheless, to design an appropriate cofactor for a given biomolecular scaffold or vice versa remains a challenge in developing efficient catalysts in biochemistry. Herein, we extend the idea of G-quadruplex-targeting anticancer drug design to construct a G-quadruplex DNA metalloenzyme. We found that a series of terpyridine–Cu(II) complexes (CuLn) can serve as excellent cofactors to dock with human telemetric G-quadruplex DNA. The resulting G-quadruplex DNA metalloenzyme utilising CuL1 catalyzes an enantioselective Diels–Alder reaction with enantioselectivity of >99% enantiomeric excess and about 73-fold rate acceleration compared to CuL1 alone. The terpyridine–Cu(II) complex cofactors demonstrate dual functions, both as an active site to perform catalysis and as a structural regulator to promote the folding of human telemetric G-quadruplex DNA towards excellent catalysts.

 Please wait while we load your content...
Please wait while we load your content...