Enzyme repurposing of a hydrolase as an emergent peroxidase upon metal binding†
Abstract
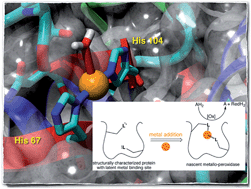
As an alternative to Darwinian evolution relying on catalytic promiscuity, a protein may acquire auxiliary function upon metal binding, thus providing it with a novel catalytic machinery. Here we show that addition of cupric ions to a 6-phosphogluconolactonase 6-PGLac bearing a putative metal binding site leads to the emergence of peroxidase activity (kcat 7.8 × 10−2 s−1, KM 1.1 × 10−5 M). Both X-ray crystallographic and EPR data of the copper-loaded enzyme Cu·6-PGLac reveal a bis-histidine coordination site, located within a shallow binding pocket capable of accommodating the o-dianisidine substrate.

 Please wait while we load your content...
Please wait while we load your content...