Counterion influence on the N–I–N halogen bond†
Abstract
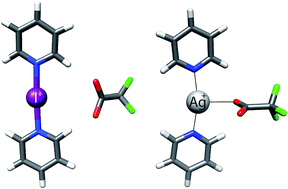
A detailed investigation of the influence of counterions on the [N–I–N]+ halogen bond in solution, in the solid state and in silico is presented. Translational diffusion coefficients indicate close attachment of counterions to the cationic, three-center halogen bond in dichloromethane solution. Isotopic perturbation of equilibrium NMR studies performed on isotopologue mixtures of regioselectively deuterated and nondeuterated analogues of the model system showed that the counterion is incapable of altering the symmetry of the [N–I–N]+ halogen bond. This symmetry remains even in the presence of an unfavorable geometric restraint. A high preference for the symmetric geometry was found also in the solid state by single crystal X-ray crystallography. Molecular systems encompassing weakly coordinating counterions behave similarly to the corresponding silver(I) centered coordination complexes. In contrast, systems possessing moderately or strongly coordinating anions show a distinctly different behavior. Such silver(I) complexes are converted into multi-coordinate geometries with strong Ag–O bonds, whereas the iodine centered systems remain linear and lack direct charge transfer interaction with the counterion, as verified by 15N NMR and DFT computation. This suggests that the [N–I–N]+ halogen bond may not be satisfactorily described in terms of a pure coordination bond typical of transition metal complexes, but as a secondary bond with a substantial charge-transfer character.


 Please wait while we load your content...
Please wait while we load your content...