Reaction-activated palladium catalyst for dehydrogenation of substituted cyclohexanones to phenols and H2 without oxidants and hydrogen acceptors†
Abstract
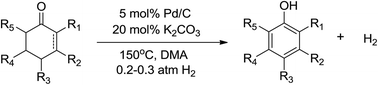
It is widely believed that the dehydrogenation of organic compounds is a thermodynamically unfavorable process, and thus requires stoichiometric oxidants such as dioxygen and metal oxides or sacrificial hydrogen acceptors to remove the hydrogen from the reaction mixture to drive the equilibrium towards the products. Here we report a previously unappreciated combination of common commercial Pd/C and H2 which dehydrogenates a wide range of substituted cyclohexanones and 2-cyclohexenones to their corresponding phenols with high isolated yields, with H2 as the only byproduct. The reaction requires no oxidants or hydrogen acceptors because instead of removing the generated hydrogen with oxidants or hydrogen acceptors, we demonstrated it can be used as a cocatalyst to help power the reaction. This method for phenol synthesis manifests a high atom economy, and is inherently devoid of the complications normally associated with oxidative dehydrogenations.

 Please wait while we load your content...
Please wait while we load your content...