What causes extended layering of ionic liquids on the mica surface?†
Abstract
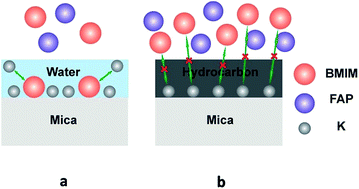
Extended layering of ionic liquids (ILs) on the mica surface has been reported by several groups previously and it is generally accepted that the electrostatic interaction at the IL/mica interface is critical to the observed extended layering. Here we report that, indeed, water adsorption on the mica surface is the key to the extended layering of ionic liquids. The atomic force microscopy (AFM), attenuated total reflectance-fourier transform infrared spectroscopy (ATR-FTIR) and contact angle (CA) results show that ionic liquids form extended layering on a mica surface under ambient conditions when water is adsorbed on the mica surface under such conditions. However, when airborne hydrocarbon contaminants replace the water on the mica surface at the elevated temperatures, instead of layering, ionic liquids exhibit droplet structure, i.e., dewetting. Based on the experimental results, we propose that water enables ion exchange between K+ and the cations of ILs on the mica surface and thus triggers the ordered packing of cations/anions in ILs, resulting in extended layering.

 Please wait while we load your content...
Please wait while we load your content...