Nitrogen fixation catalyzed by ferrocene-substituted dinitrogen-bridged dimolybdenum–dinitrogen complexes: unique behavior of ferrocene moiety as redox active site†
Abstract
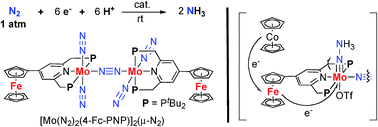
A series of dinitrogen-bridged dimolybdenum–dinitrogen complexes bearing metallocene-substituted PNP-pincer ligands is synthesized by the reduction of the corresponding monomeric molybdenum–trichloride complexes under 1 atm of molecular dinitrogen. Introduction of ferrocene as a redox-active moiety to the pyridine ring of the PNP-pincer ligand increases the catalytic activity for the formation of ammonia from molecular dinitrogen, up to 45 equiv. of ammonia being formed based on the catalyst (22 equiv. of ammonia based on each molybdenum atom of the catalyst). The time profile for the catalytic reaction reveals that the presence of the ferrocene unit in the catalyst increases the rate of ammonia formation. Electrochemical measurement and theoretical studies indicate that an interaction between the Fe atom of the ferrocene moiety and the Mo atom in the catalyst may play an important role to achieve a high catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...