Copper-catalyzed intermolecular C(sp3)–H bond functionalization towards the synthesis of tertiary carbamates†‡
Abstract
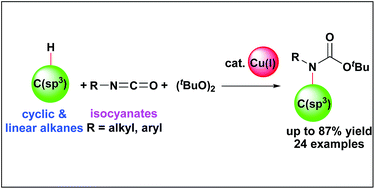
We describe the development of an intermolecular unactivated C(sp3)–H bond functionalization towards the direct synthesis of tertiary carbamates. The transformation proceeded using a readily available, abundant first-row transition metal catalyst (copper), and isocyanates as the source of the amide moiety. This is a novel strategy for direct transformation of a variety of unactivated hydrocarbon feedstocks to N-alkyl-N-aryl and N,N-dialkyl carbamates without pre-functionalization or installation of a directing group. The reaction had a broad substrate scope with 3° > 2° > 1° site selectivity. The reaction proceeded even on a gram scale, and a corresponding free amine was directly obtained when the reaction was performed at high temperature. Kinetic studies suggested that radical-mediated C(sp3)–H bond cleavage was the rate-determining step.

 Please wait while we load your content...
Please wait while we load your content...