Taming C60 fullerene: tuning intramolecular photoinduced electron transfer process with subphthalocyanines†
Abstract
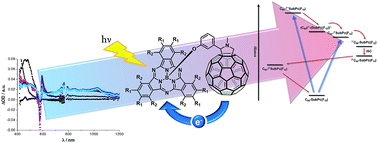
Two subphthalocyanine–C60 conjugates have been prepared by means of the 1,3-dipolar cycloaddition reaction of (perfluoro) or hexa(pentylsulfonyl) electron deficient subphthalocyanines to C60. Comprehensive assays regarding the electronic features – in the ground and excited state – of the resulting conjugates revealed energy and electron transfer processes upon photoexcitation. Most important is the unambiguous evidence – in terms of time-resolved spectroscopy – of an ultrafast oxidative electron transfer evolving from C60 to the photoexcited subphthalocyanines. This is, to the best of our knowledge, the first case of an intramolecular oxidation of C60 within electron donor–acceptor conjugates by means of only photoexcitation.

 Please wait while we load your content...
Please wait while we load your content...