Ligand-directed dibromophenyl benzoate chemistry for rapid and selective acylation of intracellular natural proteins†
Abstract
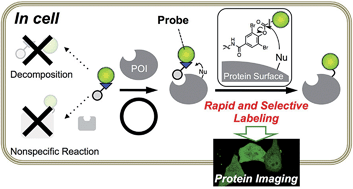
A rapid and selective ligand-directed chemical reaction was developed for the acylation of proteins in living cells on the basis of ligand-directed chemistry. By fine tuning the reactivity and stability of the phenyl ester derivatives, we successfully identified ortho-dibromophenyl benzoate as the optimal reactive motif. It was sufficiently stable in an aqueous buffer, hydrolyzing less than 10% after 13 h of incubation, but reactive enough for efficient and selective protein labeling in living mammalian cells, as well as in vitro (referred to as ligand-directed dibromophenyl benzoate (LDBB) chemistry). Using this chemistry, various fluorophores can be tethered to the target protein directly, which allows fluorescence visualization of the labeled protein in live cells using different colored fluorophore groups (including coumarin, fluorescein and rhodamine). Furthermore, this labeling is applicable to not only an overexpressed protein (E. coli dihydrofolate reductase) but also endogenous human carbonic anhydrase II and XII under living cell conditions. LDBB chemistry is a new entry of ligand-directed protein labeling methods, and should be particularly useful for the imaging of natural proteins in living cells.

 Please wait while we load your content...
Please wait while we load your content...