Task-specific ionic liquid and CO2-cocatalysed efficient hydration of propargylic alcohols to α-hydroxy ketones†
Abstract
The hydration of propargylic alcohols is a green route to synthesize α-hydroxy ketones. Herein a CO2-reactive ionic liquid (IL), [Bu4P][Im], was found to display high performance for catalyzing the hydration of propargylic alcohols in the presence of atmospheric CO2, and a series of propargylic alcohols could be converted into the corresponding α-hydroxy ketones in good to excellent yields. In the IL/CO2 reaction system, CO2 served as a cocatalyst by forming α-alkylidene cyclic carbonates with propargylic alcohols, and was released via the rapid hydrolysis of the carbonates catalysed by the IL. This is the first example of the efficient hydration of propargylic alcohols under metal-free conditions.
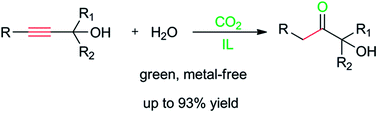

 Please wait while we load your content...
Please wait while we load your content...