Synergistic cascade catalysis by metal nanoparticles and Lewis acids in hydrogen autotransfer†
Abstract
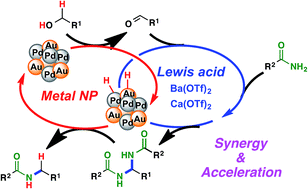
Of the many types of catalysis involving two or more catalysts, synergistic catalysis is of great interest because novel reactions or reaction pathways may be discovered when there is synergy between the catalysts. Herein, we describe a synergistic cascade catalysis, in which immobilized Au/Pd bimetallic nanoparticles and Lewis acids work in tandem to achieve the N-alkylation of primary amides to secondary amides with alcohols via hydrogen autotransfer. When Au/Pd nanoparticles were used with metal triflates, a significant rate acceleration was observed, and the desired secondary amides were obtained in excellent yields. The metal triflate is thought to not only facilitate the addition of primary amides to aldehydes generated in situ, but also enhance the returning of hydrogen from nanoparticles to hydrogen-accepting intermediates. This resulted in a more rapid turnover of the nanoparticle catalyst, and ultimately translated into an increase in the overall rate of the reaction. The two catalysts in this co-catalytic system work in a synergistic and cascade fashion, resulting in an efficient hydrogen autotransfer process.

 Please wait while we load your content...
Please wait while we load your content...