Bypassing the lack of reactivity of endo-substituted norbornenes with the catalytic rectification–insertion mechanism†
Abstract
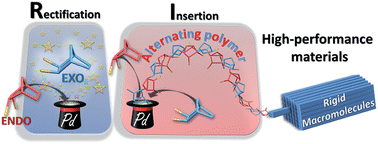
The catalytic 1,2-insertion polymerization of polar norbornenes (NBEs) leads to the formation of functional rigid macromolecules with exceptional thermal, optical and mechanical properties. However, this remarkable reaction is plagued by the low reactivity of the polar monomers, and most notably of those bearing a functional group in endo position. We have examined the polymerization mechanism of NBEs bearing one or two CO2Me groups either in exo or endo position catalyzed by the so-called naked allyl Pd+ SbF6− catalyst (1). Although endo dimethyl ester of 5-norbornene-2,3-dicarboxylic acid (NBE(CO2Me)2) is polymerized by 1, two endo units are never inserted consecutively along the polymer chain. Indeed, 1 is a tandem catalyst which not only catalyzes the insertion of the monomer but also the isomerization of endo and exo isomers. Thus, the polymerization of endo monomers proceeds via a novel mechanism, coined rectification–insertion mechanism, whereby half of the endo monomers are rectified into exo ones prior insertion, leading to the formation of an alternating endo–exo copolymer using an endo only feedstock. With this mechanism, the lack of reactivity of endo norbornenes is bypassed, and the polymerization of predominantly endo polar NBEs bearing a variety of functionalities such as esters, imides, acids, aldehydes, alcohols, anhydrides, or alkyl bromides proceeds with catalyst loadings as low as 0.002 mol%.

 Please wait while we load your content...
Please wait while we load your content...