Orthoester exchange: a tripodal tool for dynamic covalent and systems chemistry†
Abstract
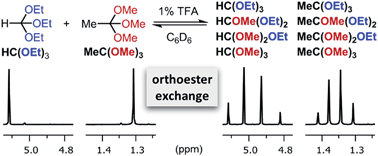
Reversible covalent reactions have become an important tool in supramolecular chemistry and materials science. Here we introduce the acid-catalyzed exchange of O,O,O-orthoesters to the toolbox of dynamic covalent chemistry. We demonstrate that orthoesters readily exchange with a wide range of alcohols under mild conditions and we disclose the first report of an orthoester metathesis reaction. We also show that dynamic orthoester systems give rise to pronounced metal template effects, which can best be understood by agonistic relationships in a three-dimensional network analysis. Due to the tripodal architecture of orthoesters, the exchange process described herein could find unique applications in dynamic polymers, porous materials and host–guest architectures.

 Please wait while we load your content...
Please wait while we load your content...