Mechanistic analysis of an asymmetric palladium-catalyzed conjugate addition of arylboronic acids to β-substituted cyclic enones†
Abstract
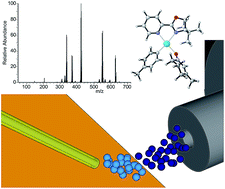
An asymmetric palladium-catalyzed conjugate addition reaction of arylboronic acids to enone substrates was investigated mechanistically. Desorption electrospray ionization coupled to mass spectrometry was used to identify intermediates of the catalytic cycle and delineate differences in substrate reactivity. Our findings provide evidence for the catalytic cycle proceeding through formation of an arylpalladium(II) cation, subsequent formation of an arylpalladium–enone complex, and, ultimately, formation of the new C–C bond. Reaction monitoring in both positive and negative ion modes revealed that 4-iodophenylboronic acid formed a relatively stable trimeric species under the reaction conditions.

 Please wait while we load your content...
Please wait while we load your content...