Magnetic circular dichroism and computational study of mononuclear and dinuclear iron(iv) complexes†
Abstract
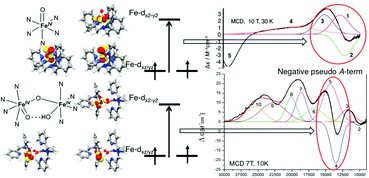
High-valent iron(IV)-oxo species are key intermediates in the catalytic cycles of a range of O2-activating iron enzymes. This work presents a detailed study of the electronic structures of mononuclear ([FeIV(O)(L)(NCMe)]2+, 1, L = tris(3,5-dimethyl-4-methoxylpyridyl-2-methyl)amine) and dinuclear ([(L)FeIV(O)(μ-O)FeIV(OH)(L)]3+, 2) iron(IV) complexes using absorption (ABS), magnetic circular dichroism (MCD) spectroscopy and wave-function-based quantum chemical calculations. For complex 1, the experimental MCD spectra at 2–10 K are dominated by a broad positive band between 12 000 and 18 000 cm−1. As the temperature increases up to ∼20 K, this feature is gradually replaced by a derivative-shaped signal. The computed MCD spectra are in excellent agreement with experiment, which reproduce not only the excitation energies and the MCD signs of key transitions but also their temperature-dependent intensity variations. To further corroborate the assignments suggested by the calculations, the individual MCD sign for each transition is independently determined from the corresponding electron donating and accepting orbitals. Thus, unambiguous assignments can be made for the observed transitions in 1. The ABS/MCD data of complex 2 exhibit ten features that are assigned as ligand-field transitions or oxo- or hydroxo-to-metal charge transfer bands, based on MCD/ABS intensity ratios, calculated excitation energies, polarizations, and MCD signs. In comparison with complex 1, the electronic structure of the FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O site is not significantly perturbed by the binding to another iron(IV) center. This may explain the experimental finding that complexes 1 and 2 have similar reactivities toward C–H bond activation and O-atom transfer.
O site is not significantly perturbed by the binding to another iron(IV) center. This may explain the experimental finding that complexes 1 and 2 have similar reactivities toward C–H bond activation and O-atom transfer.

 Please wait while we load your content...
Please wait while we load your content...