Platinum-decorated carbon nanotubes for hydrogen oxidation and proton reduction in solid acid electrochemical cells†
Abstract
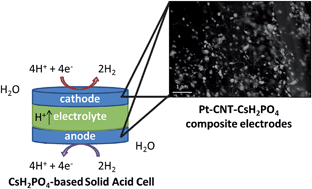
Pt-decorated carbon nanotubes (Pt-CNTs) were used to enhance proton reduction and hydrogen evolution in solid acid electrochemical cells based on the proton-conducting electrolyte CsH2PO4. The carbon nanotubes served as interconnects to the current collector and as a platform for interaction between the Pt and CsH2PO4, ensuring minimal catalyst isolation and a large number density of active sites. Particle size matching was achieved by using electrospray deposition to form sub-micron to nanometric CsH2PO4. A porous composite electrode was fabricated from electrospray deposition of a solution of Pt-CNTs and CsH2PO4. Using AC impedance spectroscopy and cyclic voltammetry, the total electrode overpotential corresponding to proton reduction and hydrogen oxidation of the most active electrodes containing just 0.014 mg cm−1 of Pt was found to be 0.1 V (or 0.05 V per electrode) at a current density of 42 mA cm−2 for a measurement temperature of 240 °C and a hydrogen-steam atmosphere. The zero bias electrode impedance was 1.2 Ω cm2, corresponding to a Pt utilization of 61 S mg−1, a 3-fold improvement over state-of-the-art electrodes with a 50× decrease in Pt loading.
- This article is part of the themed collection: Global Energy Challenges: Electrochemical Energy

 Please wait while we load your content...
Please wait while we load your content...