A combined magnetic circular dichroism and density functional theory approach for the elucidation of electronic structure and bonding in three- and four-coordinate iron(ii)–N-heterocyclic carbene complexes†
Abstract
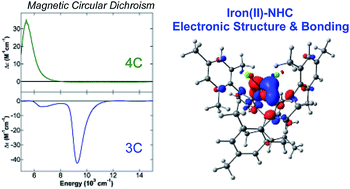
Iron salts and N-heterocyclic carbene (NHC) ligands is a highly effective combination in catalysis, with observed catalytic activities being highly dependent on the nature of the NHC ligand. Detailed spectroscopic and electronic structure studies have been performed on both three- and four-coordinate iron(II)–NHC complexes using a combined magnetic circular dichroism (MCD) and density functional theory (DFT) approach that provide detailed insight into the relative ligation properties of NHCs compared to traditional phosphine and amine ligands as well as the effects of NHC backbone structural variations on iron(II)–NHC bonding. Near-infrared MCD studies indicate that 10Dq(Td) for (NHC)2FeCl2 complexes is intermediate between those for comparable amine and phosphine complexes, demonstrating that such iron(II)–NHC and iron(II)–phosphine complexes are not simply analogues of one another. Theoretical studies including charge decomposition analysis indicate that the NHC ligands are slightly stronger donor ligands than phosphines but also result in significant weakening of the Fe–Cl bonds compared to phosphine and amine ligands. The net result is significant differences in the d orbital energies in four-coordinate (NHC)2FeCl2 complexes relative to the comparable phosphine complexes, where such electronic structure differences are likely a significant contributing factor to the differing catalytic performances observed with these ligands. Furthermore, Mössbauer, MCD and DFT studies of the effects of NHC backbone structure variations (i.e. saturated, unsaturated, chlorinated) on iron–NHC bonding and electronic structure in both three- and four-coordinate iron(II)–NHC complexes indicate only small differences as a function of backbone structure, that are likely amplified at lower oxidation states of iron due to the resulting decrease in the energy separation between the occupied iron d orbitals and the unoccupied NHC π* orbitals.

 Please wait while we load your content...
Please wait while we load your content...