Abstract
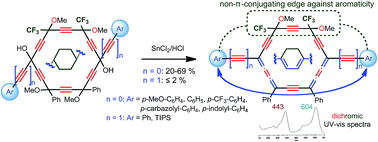
Ideally Cs-/C2v-symmetric chromophores, constituted by two electro-active groups conjugated through the carbo-mer of the cyclohexa-1,3-diene core, are selectively prepared by the SnCl2-mediated reduction of tailored hexaoxy-[6]pericyclynes: in the latter substrates, one of the 1,4-dioxybut-2-yne edges is “chemically locked” by two CF3 substituents preventing complete reduction to the corresponding aromatic carbo-benzenic core, which is expected to be more “π-insulating” between the electro-active ends. The bis-trifluoromethylated carbo-cyclohexadiene products are also shown to be significantly stabilized with respect to their bis-phenylated analogues. Their structural (crystal X-ray diffraction analyses), spectroscopical (NMR and UV-vis spectra), physio-optical (dichromism in solution) and electrochemical (cyclic voltammograms) properties are compared on the basis of the electron-donating/electron-withdrawing nature of the substituents. These properties are also compared with those of their aromatic carbo-benzene and flexible carbo-n-butadiene counterparts.

 Please wait while we load your content...
Please wait while we load your content...