Electron localization in a mixed-valence diniobium benzene complex†
Abstract
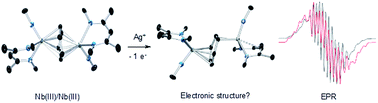
Reaction of the neutral diniobium benzene complex {[Nb(BDI)NtBu]2(μ-C6H6)} (BDI = N,N′-diisopropylbenzene-β-diketiminate) with Ag[B(C6F5)4] results in a single electron oxidation to produce a cationic diniobium arene complex, {[Nb(BDI)NtBu]2(μ-C6H6)}{B(C6F5)4}. Investigation of the solid state and solution phase structure using single-crystal X-ray diffraction, cyclic voltammetry, magnetic susceptibility, and multinuclear NMR spectroscopy indicates that the oxidation results in an asymmetric molecule with two chemically inequivalent Nb atoms. Further characterization using density functional theory (DFT) calculations, UV-visible, Nb L3,2-edge X-ray absorption near-edge structure (XANES), and EPR spectroscopies supports assignment of a diniobium complex, in which one Nb atom carries a single unpaired electron that is not largely delocalized on the second Nb atom. During the oxidative transformation, one electron is removed from the δ-bonding HOMO, which causes a destabilization of the molecule and formation of an asymmetric product. Subsequent reactivity studies indicate that the oxidized product allows access to metal-based chemistry with substrates that did not exhibit reactivity with the starting neutral complex.

 Please wait while we load your content...
Please wait while we load your content...