α-CH acidity of alkyl–B(C6F5)2 compounds – the role of stabilized borata-alkene formation in frustrated Lewis pair chemistry†
Abstract
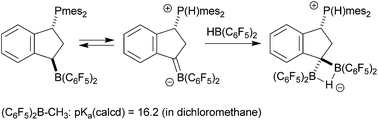
Alkyl–B(C6F5)2 boranes are markedly α-CH-acidic. Using DFT we have calculated the pKa-values of a series of examples. Typically, (C6F5)2B–CH3 [pKa (calcd) = 18.3 in DMSO, 16.2 in dichloromethane] is almost as CH-acidic as cyclopentadiene. However, this α-CH–B(C6F5)2 acidity is in most cases not sufficient to allow for internal proton transfer in vicinal phosphane/borane frustrated Lewis pairs (FLPs). An exception is the slightly endergonic (+0.3 kcal mol−1) tautomerization of the in situ generated indane derived 1,3-P/B FLP 6 to its zwitterionic borata-alkene/phosphonium isomer 7, which was successfully trapped by Piers' borane [HB(C6F5)2] to yield the stable product 8. The pronounced α-CH[B] carbanion stabilization can be used synthetically. We have found that the dienyl borane E-H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Me)CH
C(Me)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHB(C6F5)2 undergoes clean, thermally induced 1,4-hydrophosphination reactions with HPR2 (R: phenyl, mesityl, or t-butyl) reagents. α-CHB(C6F5)2 carbanion (i.e. borata-alkene) stabilization in the respective intermediates probably plays a decisive role in these reactions.
CHB(C6F5)2 undergoes clean, thermally induced 1,4-hydrophosphination reactions with HPR2 (R: phenyl, mesityl, or t-butyl) reagents. α-CHB(C6F5)2 carbanion (i.e. borata-alkene) stabilization in the respective intermediates probably plays a decisive role in these reactions.

 Please wait while we load your content...
Please wait while we load your content...