Synergistic effects of halogen bond and π–π interactions in thiophene-based building blocks†
Abstract
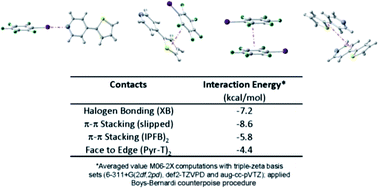
Although recognized as a significant force in crystal engineering, halogen bonding (XB) has been scarcely investigated in “bottom-up” approaches towards organic electronics. We report, herein, the use of a thiophene-based building block, pyridyl-thiophene (Pyr-T), to achieve an assembly driven by XB and π–π stacking interactions with iodopentafluorobenzene (IPFB). Spectroscopic and thermal analysis of the co-crystal provide indirect evidence of the assembly. The combined effects of XB and π–π stacking are confirmed experimentally via X-ray crystallography. Density functional theory (DFT) computations support experimental observations. The results of the study speak to the use of halogen bond driven self-assembly in organic electronic device application.

 Please wait while we load your content...
Please wait while we load your content...