Enantioselective addition of oxazolones to N-protected imines catalysed by chiral thioureas†
Abstract
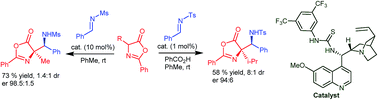
Hydrogen bond-catalysed aza-Mannich addition of oxazolones to various protected aldimines has been developed. The resulting, highly functionalized, oxazolones contain a quaternary stereogenic centre and can serve as precursors for chiral α,β-diamino acids with different protecting groups on each amino group. The process benefits from the versatile bifunctional thiourea catalysts, which effect the formation of these products in high yields and stereoselectivities.

 Please wait while we load your content...
Please wait while we load your content...