Synthesis of optically pure (S)-2-amino-5-arylpent-4-ynoic acids by Sonogashira reactions and their potential use as highly selective potent inhibitors of aldose reductase†
Abstract
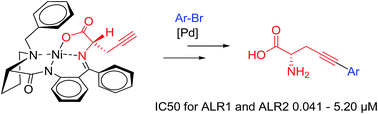
A new and convenient synthesis of optically pure (S)-2-amino-5-[aryl]pent-4-ynoic acids (alkynylated amino acids) is reported. (S)-2-Aminopent-4-ynoic acid-Ni-(S)-BPB was prepared and then functionalized via Sonogashira cross-coupling reactions to give (S)-2-amino-5-[aryl]pent-4-ynoic acid-Ni-(S)-BPB. The reaction conditions for the cross-coupling reaction were optimized and twelve derivatives were synthesized. Afterwards the Ni-complexes were cleaved to obtain the free (S)-2-amino-5-[aryl]pent-4-ynoic acids with excellent optical purity. The prepared (S)-2-amino-5-[aryl]pent-4-ynoic acids were tested for biological activity and selectively showed high inhibitory activity against aldose reductase (ALR2) over ALR1. Molecular docking studies have also been carried out to identify the putative binding mode of the compounds in these enzymes.

 Please wait while we load your content...
Please wait while we load your content...