Facile catalyst-free synthesis of 2-vinylquinolines via a direct deamination reaction occurring during Mannich synthesis†
Abstract
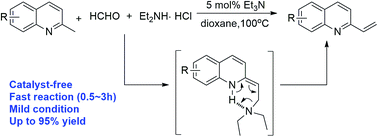
A facile catalyst-free method for the synthesis of 2-vinylquinolines via a direct deamination reaction during Mannich synthesis has been developed. Instantaneous hydrogen transfer via a six-membered ring intermediate is proposed as a key step for the direct deamination reaction. This reaction strategy tolerates a broad substrate scope and provides a highly efficient way to synthesize 2-vinylquinolines with adequate yields.

 Please wait while we load your content...
Please wait while we load your content...