Halogen-doping in LiCoO2 cathode materials for Li-ion batteries: insights from ab initio calculations
Abstract
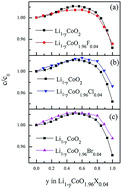
Lithium cobalt oxide is one of the most commonly used cathode materials for Li ion batteries. However, the electrochemical cycling performance is limited by the structural instability of LiCoO2 during the charging/discharging processes. Using density functional theory calculations, we investigate the effects of halogen doping on the structural stability, electronic state, electrode potential, and Li diffusion behavior of LiCoO2 systems. Fluorine, chlorine, and bromine substitutions of oxygen species suppress the lattice changes upon Li deintercalation, enhance the structural stability, electronic conductivity and Li mobility, as well as retain the electrode potential of the undoped system. Thus, halogen doping opens an effective route to improve the structural and electrochemical properties of LiCoO2 cathodes for Li ion batteries with better rate capacities and longer lifetimes.

 Please wait while we load your content...
Please wait while we load your content...