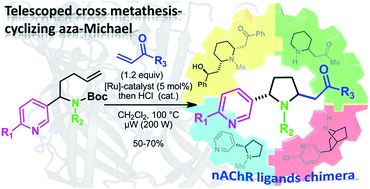
Microwave-assisted telescoped cross metathesis-ring closing aza-Michael reaction sequence: step-economical access to nicotine–lobeline hybrid analogues†
Abstract
A series of 2,5-disubstituted pyrrolidines was synthesized through an efficient telescoped cross-metathesis/cyclizing aza-Michael addition involving N-heteroaromatic olefinic derivatives. This synthetic route was applied to the preparation of original nicotine–lobeline, nicotine–pelletierine and lobeline–nicotine–epibatidine hybrids.

 Please wait while we load your content...
Please wait while we load your content...