A validated chromatographic method for simultaneous determination of guaifenesin enantiomers and ambroxol HCl in pharmaceutical formulation
Abstract
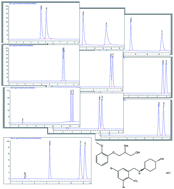
The performance of three phenylcarbamate based chiral stationary phases was evaluated for the optimum separation of guaifenesin enantiomers. Resolution, enantioselectivity and capacity factors were compared simultaneously using four factor three level experimental design. Chiralcel OD provided the highest resolution and selectivity but the lowest capacity factor for the less retained enantiomer along with peak broadening for the more retained enantiomer. On the other hand, Lux amylose-2 provided the lowest parameters. Optimum resolution and selectivity with the highest capacity factors was provided by Lux cellulose-2 as stationary phase and ethanol/hexane (15 : 85 v/v) as a mobile phase at a flow rate of 1.2 mL min−1 and column temperature at 19 °C. Extended separation of guaifenesin enantiomers and ambroxol HCl was accomplished using the same optimized chromatographic conditions. The proposed methods were applied for the determination of analytes in syrup formulation with high specificity. The method was validated as per International Conference on Harmonization guidelines and compared with a reported HPLC method.

 Please wait while we load your content...
Please wait while we load your content...