Synthesis, structure and magnetic characterization of dinuclear copper(ii) complexes bridged by bicompartmental phenolate†
Abstract
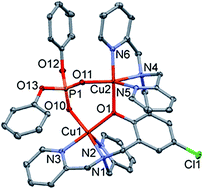
The reaction of Cu(II) salts with the bicompartmental 2,6-bis[bis(2-pyridylmethyl)aminomethyl]-4-chlorophenol (LCl-OH) ligand afforded four new dinuclear bridged phenoxido Cu(II) complexes. Three doubly bridged complexes namely [Cu2(μ-LCl-O)(μ-X)](ClO4)2 (1: X = OH−, 3: X = O2P(OC6H5)2−) and 2: [Cu2(μ-LCl-O)(μ-pz)(ClO4)]ClO4 (2) where pz = pyrazolyl anion, and one singly bridged-phenoxido, [Cu2(μ-LCl-O)(dca)2]PF6·2CH3CN (4·2CH3CN) (dca = dicyanamide anion). A complex similar to 4 was also obtained with 2,6-bis[bis(2-pyridylmethyl)aminomethyl]-4-methylphenol (LMe-OH), [Cu2(μ-LMe-O)(dca)2]PF6·2CH3CN (5·2CH3CN) where in both cases dca are acting as terminal monodentate ligands. The complexes were structurally characterized by various spectroscopic techniques (IR, UV-VIS and ESI-MS) and by single crystal crystallography. Magnetic susceptibility measurements at variable temperature revealed strong to very strong antiferromagnetic coupling (AF) in the doubly bridged complexes 1–3 and very weak AF interaction in the dicyanamido compounds 4 and 5. The DFT calculations for the coupling constants, J were in agreement with the experimentally observed behavior. The trend in magnetic properties was attributed to the strength of overlap between the orbitals (dx2−y2/dx2−y2vs. dz2/dx2−y2vs. dz2/dz2) resulting from trigonal bipyramidal (TBP) or square pyramidal (SP) geometries.


 Please wait while we load your content...
Please wait while we load your content...