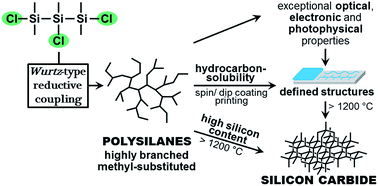
Synthesis of hydrocarbon-soluble, methyl-substituted highly branched polysilanes via the Wurtz-type reductive coupling of trifunctional trisilanes and their pyrolysis to silicon carbide†
Abstract
A hydrocarbon-soluble, methyl-substituted highly branched polysilane was synthesized by subjecting a new type of monomer to the common Wurtz-type reductive coupling reaction conditions. For this purpose, the trichloro-substituted trisilane (ClMe2Si)2SiMeCl (1) was used as a monomer. Polymerization was conducted at elevated temperature using dispersed sodium in toluene. Termination was initiated after 4 h by the addition of MeMgBr and precipitation in MeOH. The branched polysilane was analyzed by GPC, NMR and IR spectroscopy after quenching with MeMgBr and additionally after precipitation in MeOH. Opto-electronic properties were tested by UV/Vis and PL spectroscopy. Thermal properties were examined via TGA and DSC. The synthesized polysilane is usable as a soluble precursor for the preparation of SiC, not least due to its high silicon content. The chemical process during pyrolysis was examined at different temperatures by means of 1H NMR, IR and UV/Vis spectroscopy. XRPD analysis, IR spectroscopy, HR-SEM-EDS and elemental analysis confirmed the successful formation of β-SiC.

 Please wait while we load your content...
Please wait while we load your content...