A facile cosolvent/chelation method for the preparation of semi-crystalline CuCl2(ethylene glycol)/poly(3-hexylthiophene) complexes displaying specific luminescence properties
Abstract
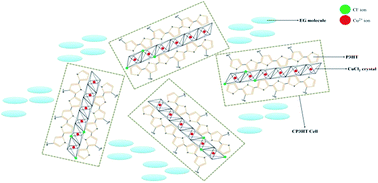
Semi-crystalline CuCl2(ethylene glycol)/poly(3-hexylthiophene) complexes (CE–P3HT) with specific textures and self-assembled structures can be fabricated using a facile co-solvent/chelation method. The morphologies and self-assembled structures of these CE–P3HT complexes were dependent on the concentration of CuCl2(ethylene glycol) (CuCl2EG) in solution. Transmission electron microscopy (TEM) revealed the CE–P3HT sample comprising EG and numerous CuCl2/P3HT dots (C–P3HT dots) that originated from the P3HT main chains interacting with CuCl2 crystals; the average size of this C–P3HT dots was ca. 20 nm. Prior to formation of the solid state of CE–P3HT structures, the CE–P3HT complex in solution displayed a novel phenomenon: a specific luminescence property with an absorption energy gap of 3.71–4.46 eV. This quantum effect resulted from the chelates formed between the Cu2+ ions and the thioether units of the conjugated P3HT polymer.

 Please wait while we load your content...
Please wait while we load your content...