PhI(OCOCF3)2-mediated ruthenium catalyzed highly site-selective direct ortho-C–H monoarylation of 2-phenylpyridine and 1-phenyl-1H-pyrazole and their derivatives by arylboronic acids†
Abstract
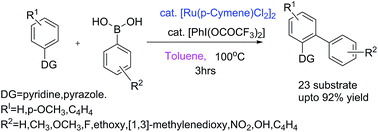
We report a concise, versatile, and practical method for PhI(OCOCF3)2 mediated direct ortho C–H monoarylation of 2-phenylpyridine and its derivatives and 1-phenyl-1H-pyrazoles via Ru catalyzed reaction. The significant advantage of this transformation is the creation of highly site selective C–C bond by using arylboronic acids as arylating agent under mild reaction conditions.

 Please wait while we load your content...
Please wait while we load your content...