Formal total synthesis of calothrixin B and its N-benzyl analogues†
Abstract
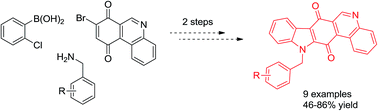
A formal total synthesis of calothrixin B and its N-benzyl analogues has been accomplished from (2-chlorophenyl)boronic acid, 8-bromophenanthridine-7,10-dione and benzylamines. The synthesis involves Suzuki cross-coupling and copper-catalyzed domino reactions to generate N-benzylcalothrixins in good to moderate yields.

 Please wait while we load your content...
Please wait while we load your content...