Vinyl benzoxazine: a novel heterobifunctional monomer that can undergo both free radical polymerization and cationic ring-opening polymerization
Abstract
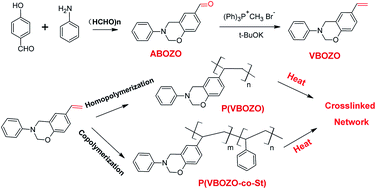
A novel heterobifunctional monomer, namely (6-ethenyl-3-phenyl-3,4-dihydro-2H-1,3-benzoxazine (VBOZO)), which contains both a vinyl group and benzoxazine group, was successfully prepared using the normal Wittig method from an aldehyde-containing benzoxazine precursor of 3-phenyl-3,4-dihydro-2H-1,3-benzoxazine-6-carbaldehyde. VBOZO underwent free radical polymerization initiated by azodiisobutyronitrile (AIBN) to form a homopolymer P(VBOZO) with a number-average molecular weight of about 5050 g mol−1. Meanwhile the benzoxazine group remains unchanged in the copolymer and can undergo cationic polymerization at high temperature to form crosslinked polymers. Benzoxazine-containing linear P(VBOZO) has a Tg of about 125 °C and shows much better thermal stability than polystyrene. The peak temperature assigned to the cationic ring-opening polymerization of P(VBOZO) was centered at 263.0 °C. The VBOZO also copolymerized with styrene under free radical conditions to get the copolymer P(VBOZO-co-St). The polymerization character of VBOZO is very similar to styrene and the copolymer composition is very close to the feed ratio of monomers. All copolymers show high thermal stability and the char yields at 800 °C are over 20%.

 Please wait while we load your content...
Please wait while we load your content...