The use of polymer-supported Candida antarctica lipase B to achieve the entropically-driven ring-opening polymerization of macrocyclic bile acid derivatives via transesterification: selectivity of the reactions and the structures of the polymers produced†
Abstract
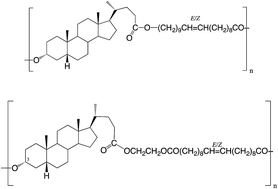
Various macrocyclic lactones containing bile acid residues successfully undergo entropically-driven ring-opening polymerization via transesterifications (TEs) catalyzed by polymer-supported Candida antarctica lipase B (PS-CALB) when the 3α- and 24-positions are linked by C14 or C20 chains or by a 23-atom chain, but not when the 3α- and 12α-positions are linked by a C20 chain. This suggests that the α-face of the bile acid derivatives in the region of the A–C rings sit at the active site of the enzyme. TEs of model bile acid derivatives indicate that the order of reactivity of ester groups catalyzed by CALB is 3α-OAc > 26-OAc ≫ 24-CO2R, though the latter do react. The 12α-OH does not react. Accordingly, under the polymerization conditions used in this work, the polymeric products formed from the macrocyclic bile acid derivatives containing a C14 or C20 linkage between the 3α- and 24-positions are expected to be a polymer chain formed essentially by ring-opening at the 3α-position, with the repeat units arranged in a regular head-to-tail manner. This differs from the structure of the polymers obtained by ED-ROMP of the same macrocycles. The macrocycles formed formally from glycol lithocholate (the diol unit) and E/Z-eicos-10-enedioic acid (the diacid unit) afford polymers that essentially have these units linked in an alternating manner. There are regiosiomers because of the unsymmetrical diol unit. Esterifications and TEs catalyzed by PS-CALB using a large excess of appropriate volatile aliphatic esters are a useful alternative to more expensive activated esters in the synthesis of esters using PS-CALB.

 Please wait while we load your content...
Please wait while we load your content...