Acetone alkylation with ethanol over multifunctional catalysts by a borrowing hydrogen strategy
Abstract
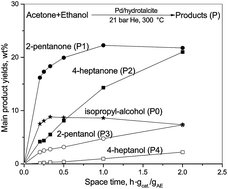
Step by step alkylation of acetone (A) with ethanol (E) in a ratio of 1 : 2 was investigated. A fixed bed flow-through reactor system was used at a total pressure of 21 bar and in the temperature range of 150–350 °C in inert He or a reducing H2 medium. Following the hydrogen borrowing methodology, two types of catalysts were prepared; using neutral activated carbon (AC) and alkaline hydrotalcite (HT) supports, namely 5 wt% Pd/AC in the presence of alkaline additives (10, 20 and 30 wt% KOH or 20% K3PO4); 9 wt% Cu/HT and 5 wt% Pd/HT. The catalysts were activated in a H2 flow at 350 °C. Different yields of mono- or dialkylated ketones were observed. In a hydrogen medium over the same catalyst systems the ketone products could be reduced to alcohols. In this study the Pd/HT catalyst seems to be the most promising for fuel production based on biomass fermentation.


 Please wait while we load your content...
Please wait while we load your content...