Solubility and solution thermodynamics of rhein in eight pure solvents from (288.15 to 313.15) K†
Abstract
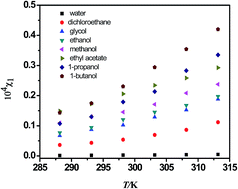
Data on solid + liquid equilibrium of rhein in solvents can provide essential support for the design of extraction, precipitation, or crystallization processes in industry. In this study the solubility of rhein in eight different solvents namely water, 1,2-dichloroethane, glycol, ethanol, methanol, ethyl acetate, 1-propanol, and 1-butanol was measured over a temperature range from 288.15 to 313.15 K under atmospheric pressure using a UV spectrophotometric method. The experimental result shows that 1-butanol, 1-propanol and ethyl acetate could be suitable solvents for the industrial production of rhein. Two commonly used thermodynamic equations, including the modified Apelblat equation, and λh equation were applied to correlate the experimental solubility data. It was found that the two equations can satisfactorily correlate the solubilities of rhein in the eight solvents under different temperatures. The dissolution enthalpy and entropy were obtained from the experimental values. The dissolution process of rhein in eight solvents within the studied temperature range was endothermic, and the driving mechanism is the entropy.

 Please wait while we load your content...
Please wait while we load your content...